Stainless Steel Tubing corrosion
Stainless steel tubing in oil and gas use in process instrumentation with range of temperature, flow rate and pressure conditions.
General Process Corrosion
- Corrosion rate is less than 0.1 mm/y is normally consider resistant
- Higher the concentration, and temperature of the solution, is more aggressive it becomes
- Impurities can have a drastic influence.

Erosion Corrosion
- Corrosion can occur when the material is simultaneously expose to corrosion high, flow rates and also mechanical wear.
- The presence of solid particles can speeds up the erosion corrosion rate.

Stainless Steel Screw with Cadmium Washer
Galvanic Corrosion
- Can occur when two metals or alloys contact with one or another in the presence of an electrolyte
- The less noble material will corrode.

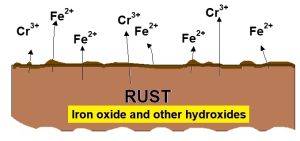
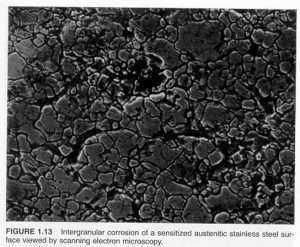
Intergranular Corrosion
Results from chromium depletion due to precipitation of chromium carbides in the grain boundaries.
Stress Corrosion Cracking (SCC)
- Cracks along the grains or grain boundaries
- Results from combined action of tensile stresses, and a corrosive medium, and elevated temperatures
- Chloride bearing, and hydrogen sulfide (sour service) environments are conducive to SCC
- 304L and 316L austenitic steels have limited resistance to SCC
- Ferritic steels, and duplex stainless steels, and austenitic with high nickel content are highly resistant to SCC

Contamination Stainless Steel Tubing is leading Surface Degradation
The two prevalent forms of localized corrosion are pitting corrosion, which is often readily recognizable, and crevice corrosion. Pitting is can be more difficult to observe. The selection of in adequate tubing alloy and sub optimal installation practices can lead to deterioration of tubing surfaces. Today’s minimally alloyed 316 stainless steel tubing, with close to 10.0% nickel, 2.0% molybdenum and 16.0% chromium.
Contamination another leading cause for surface degradation. Contamination is may be cause by iron particles come from welding and grinding operations in sea. Periodic testing of seawater deluge systems is especially in combination with insufficient freshwater cleansing and may also leave undesirable chloride laden deposits behind.

PITTING AND CREVICE CORROSION in Stainless Steel Tubing
In most cases, pitting corrosion of tubing can be readily recognized. Individual shallow pits, and in later stages, deep and sometimes connected pits , can be observed by visual inspection with the unaid eye, show in Fig-1. Pitting corrosion will starts when the chromium rich passive oxide film on 316 tubing and breaks down with a chloride rich environment. Higher chloride concentrations, and elevated temperatures is increase the likelihood for breakdown of this passive film. Once the passive film has been breach it’s will cause an electrochemical cell becomes active. so Iron goes into solution in the more anodic bottom of the pit, and diffuses toward the top, and oxidized to iron oxide, or rust. The iron chloride solution concentration in a pit can increase as the pit gets deeper.
Crevices Avoid in tubing installations
Pitting can penetrate deep into the tubing walls, and creating a situation where tubing could fail. Crevices are very difficult, or even impossible, and to avoid in tubing installations. They exist between tubing and tube supports systems, or in tubing clamps, and between adjacent tubing runs. Underneath contamination, deposits that may have accumulated on tubing surfaces. Tight crevices pose therefore the greatest danger for crevice corrosion. General tubing corrosion happen when a tight crevice causes the oxygen concentration in the fluid that contained within a crevice to drop and due to lower oxygen concentration will increases the likelihood for breakdown of the passive surface oxide film, and resulting in the formation of a shallow pit..

Pitting Corrosion
Unlike in pitting corrosion that describe above. Pit formation on tubing that is surround by a crevice will lead to an increase in Ca++ concentration in the fluid in the gap. Because of the strong interaction of Fe++ ions, with OH hydroxyl ions, and include the pH value drops. Chloride ions will also diffuse into the gap, being attract by Fe++ ions, and the resulting in an acidic ferric chloride solution. It’s that can lead to accelerated corrosion of tubing within the crevice.
Tubing should resist all forms of corrosion. Localized pitting and crevice, and galvanic, and microbiological, and chloride induced, or stress corrosion cracking, and sour gas cracking. The tubing should also have adequate mechanical properties for especially fluid high pressures. The environmental impact of the tubing should be of concern and Aquatic life can be harm by small concentrations of copper ions that can be readily released by copper zinc alloys.

Prevention of Corrosion Tubing
ALLOY SELECTION
The importance of selecting the optimal alloy for an installation is demonstrate in Fig 2. When installation side by side, austenitic 316 stainless steel tubing have experienced heavy corrosion res, that no signs of corrosion were detect on alloy 2507 super duplex tubing. In a GOM installation of alloy 2507 tubing, we can see only very small number of cases of external chloride crevice corrosion damage were identify. Perforations as the leading to the loss of system fluid containment were not observe. The only crevice corrosion damage involved in plastic support strips, and neoprene gaskets.

Fig. 2. Image shows 316 stainless steel and alloy 2507 superduplex tubing installed side by side, with the 316 tubing showing extensive corrosion and the superduplex tubingshowing none.
Alloys is used Resistance to seawater corrosion in installations.
Banyak paduan adalah kandidat untuk digunakan dalam instalasi yang membutuhkan ketahanan terhadap korosi air laut. Paduan yang paling sering digunakan adalah baja tahan karat austenitik seri 300 dengan terutama 316 dan dalam beberapa kasus 317. Paduan dengan setidaknya 6% molibdenum, dan kami sebut sebagai 6 moly, telah berkinerja baik dalam sistem lepas pantai. Paduan 6 moly khas termasuk 254SMO, AL6XN dan 25-6Mo. Baru-baru ini, paduan dengan sedikit lebih dari 6% molibdenum telah diperkenalkan: 654SMO, AL6XN Plus, 27 7Mo dan 31. Sifat-sifat yang dipublikasikan dari paduan ini menunjukkan bahwa mereka akan bekerja dengan baik di lingkungan klorida. Paduan nikel seperti 825, dan 625 dan C 276 lebih sering digunakan untuk kinerjanya dalam aplikasi gas asam, paduan ini, 625 dan C 276 telah menunjukkan ketahanan yang sangat baik terhadap korosi lokal.
Ferritic alloys like Sea Cure and AL29 4C are resistant to attack by liquid chloride solutions and the primarily used as heat exchanger tubing. Tungum is a copper zinc alloy that has been used, because of its relative ease for installation. However, it carries disadvantages that lack of hardness indicates susceptibility to erosive wear, and low yield. Strength restricts its use to low pressures or requires high wall thickness; and corrosion liberates copper ions that can be detrimental to sea life.
Duplex Alloys
The growing number of duplex alloys reflects the increasing use of this promising class of materials. The 2205 duplex alloy was introduce decades ago. Now there is super duplex alloy 2507, which has performed very well in recent years in more demanding applications that require PREN values of 40 and above. More recently, the hyper duplex alloy 3207 was introduce with an even higher PREN value. At the low end of alloy content, several lean duplex alloys such as 2101, 2304 and 2003 is present themselves as candidates for less demanding applications.
The critical pitting temperature and critical crevice temperature of several alloys are shown in Fig. 3. The increase in chromium, molybdenum and nitrogen clearly leads to an increase in the CPT and CCT values of austenitic and duplex stainless steels. Also, despite their overall lower content of nickel and molybdenum, they offer a similarly performance to that of highly alloyed austenitic stainless steels.
Duplex alloys offer satisfactory resistance to localized corrosion and have high mechanical properties, which make them best candidates for high pressure applications. Alloy 2507 has a yield strength more than three times that of 316L.
CPT and CCT values of austenitic and duplex stainless steels
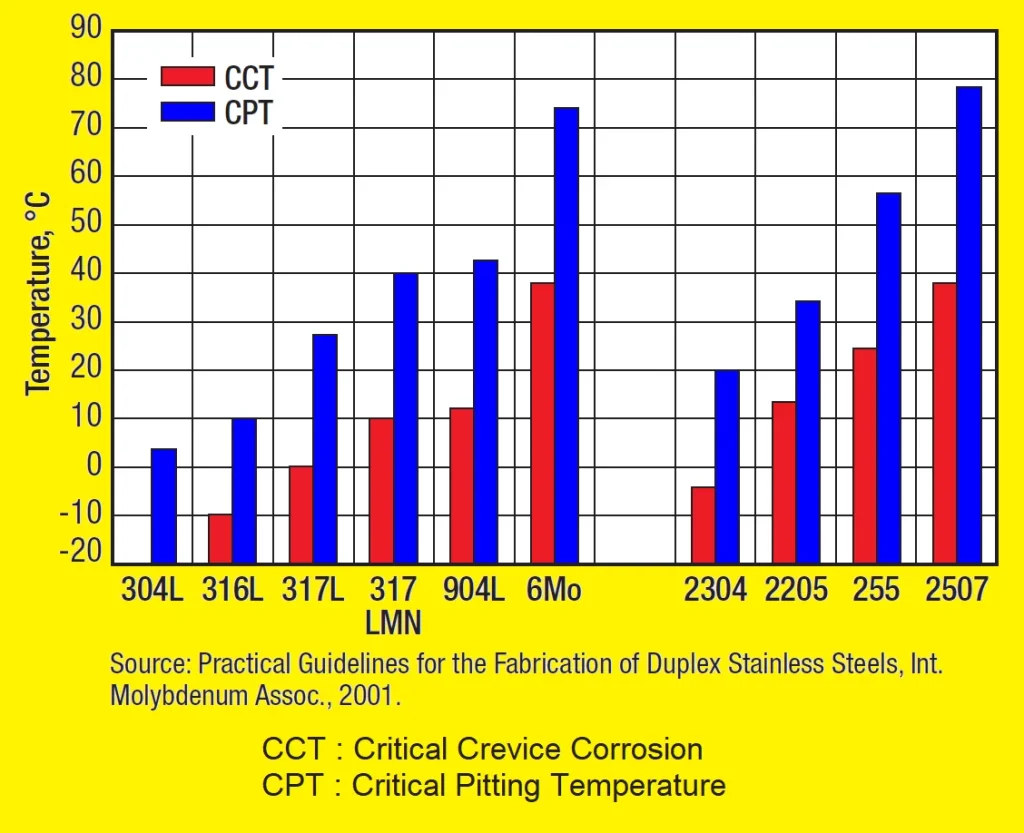
Fig. 3. Bar chart shows critical pitting temperature and critical crevice corrosion temperatures of austenitic and duplex stainless steels. Measured by ASTM G48 in 10% ferric chloride.
Isolation Tube
Coatings are usually liquid-applied coverings that are applied to surfaces in contact with corrosive situations such as the atmosphere. Coatings are used to control corrosion, and can be applied using a Jacket to coat the surface of a substrate, such as PVC. Widely used in marine environment to control Corrosion will eventually penetrate the coating so that it has a design life before treatment.
We are PT. Solutec Daya Mandiri as sole agent of Instrument Tubing and Fitting is ready provide Tubing Stainless Stell and Alloy Material.

Fig. 4. Jacket tubing is one way to reduce corrosion that occurs on tubing



